Niche Interaction
Remarkably, while the niche is required for tissue regeneration, we found that dedicated stem cells are dispensable, because when stem cells are ablated the niche can recover stem cells from other locations (Rompolas 2013; Mesa 2015).
Tracking the same dermal fibroblasts over time in live mice revealed that while individual fibroblasts have remarkably stable positions in homeostasis, neighboring cells respond to local cell loss not via proliferation, but instead by extending their membranes into the depleted space in a Rac1-dependent manner (Marsh 2018).
Recently, we have worked on understanding how fibroblast organize and support the overlying stem cell layer, how hair follicle niche fibroblasts support stem cells and overall organ function, the maturation and maintenance of small blood vessels irrigating the skin, and the influence of fibroblasts on overlying stem cell proliferation and extracellular matrix assembly.
Fibroblasts extend their membranes in response to injury and aging instead of proliferating
We also discovered that fibroblasts are remarkably positionally stable in the adult skin, in contrast to being highly dynamic in vitro, and in our ongoing work we are studying how their organization and functions as niche components to epidermal stem cells evolve and mature during postnatal development (Marsh 2018).
Timelapse movie of a single membrane-GFP labeled fibroblast in undamaged live mouse paw skin upper dermis.
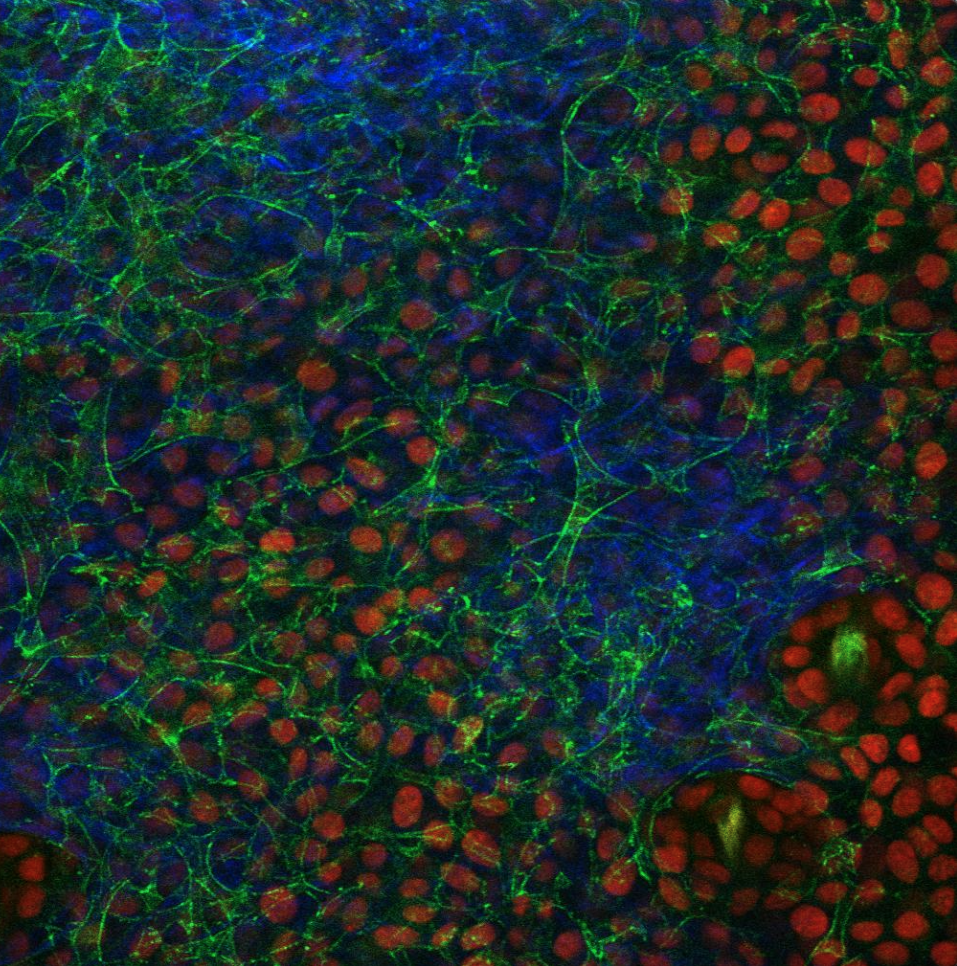
Hair follicle niche fibroblasts support stem cell function even after severe disruption of organ architecture
In the hair follicle, the dermal papilla (DP) fibroblasts serve as a niche for stem cells. We found out that the dermal papilla polarization allows for its progressive remodeling into a structurally robust unit during hair follicle growth. This architectural remodeling requires fibroblast TGFβ signaling. Removing TGFβ receptor 1 in fibroblasts results in reorganization of the niche architecture and the hair progenitor cells they were surrounding. Surprisingly, progenitor function is still largely preserved, with only mild defects resulting in shorter hair production. This study led to the development of tools needed to probe the relationship between fibroblasts and the epithelium in order to understand how this relationship changes over time under different context: postnatal development, injury and aging (Wei 2023).
Endothelial cells migrate to maintain blood vessel network integrity in neonates and self-repair once positionally stable in adulthood
Unlike epithelial stem cells, endothelial cells and fibroblasts in the dermis do not appear to rely on proliferation to maintain homeostasis. We recently showed that a fixed number of neonatal endothelial cells rearrange their positions as the vascular plexus develops, eventually becoming positionally stable in adulthood. However, upon neighboring cell loss, endothelial cells reactivate migratory and self-repair behaviors to maintain overall vascular architecture and function (Kam 2023).
Intravital image of Tgfbr1+/fl and Tgfbr1fl/fl hair follicles at mid- and late-growth stages after CreER induction at the quiescent stage. Fibroblast membranes are in green and other membranes are in red.
(Top) Tracking of an individual endothelial cell undergoing vessel regression (white asterisk) during neonatal capillary network maturation. White arrow depicts direction of cell migration.
(Bottom) Tracking of single endothelial cells (numbered) in adult mice imaged longitudinally over 1 month.