Epithelial Tolerance
By tracking oncogene-induced growths, we discovered that neighboring wild-type cells can actively eliminate mutant cells leading to growth regression, and this takes place in a non‑inflammatory setting (Deschene & Myung 2014; Brown & Pineda 2017).
Recently, we have worked on understanding the impact wounds have on oncogenic cell competition, the perturbation of tissue architecture and signaling alterations caused by oncogenic mutations and the changes in metabolism and redox ratios caused by oncogenic mutations.
Wild-type cells suppress oncogenic cells expansion following wounding
Surprisingly, we recently discovered that the injury-repair process switches the competitive balance between Hras mutant and wild-type epithelial stem cells: wild-type cells selectively increase their proliferation, thereby suppressing the expansion of Hras mutant cells and preventing tumorigenesis. Mechanistically, this switch is due to differential responses to injury-induced MAPK signaling. This opens the door to potential targeted therapeutics harnessing the power of our tissues to preserve overall architecture and homeostasis even when neighbors harbor oncogenic mutations (Gallini 2023).
Intravital images of the basal stem cell layer composed of a mosaic cell population (WT and Hras mutant cells) in uninjured (top) and injured (bottom) conditions. Epithelial nuclei are in green (Krt14-H2B–GFP) and recombined cells are in red (FR-HrasG12V/+ ; LSL-tdTomato).
ERK signaling is altered in Kras oncogenic cells, where sustained activation drives aberrant morphology
We explored the mechanisms by which oncogenes cause perturbation of tissue architecture. We found that a Kras mutation that can lead to squamous cell carcinoma, G12D, induces aberrant growths in hair follicles in a spatiotemporal specific manner. Kras mutant cells divide at abnormal angles, contributing to the formation of bump-like lateral growths. We showed, using a reporter mouse and Kras induction in a mosaic manner, that wild-type hair follicle stem cells display pulsatile ERK signaling. In contrast, Kras mutant cells show sustained ERK activation. Inhibiting MEK, a kinase upstream of ERK, in Kras mutant cells leads to less abnormal perpendicular divisions and it both prevents and reverts tissue deformation. As tissue deformations are resolved upon ERK pathway inhibition, we show that sustained activation of ERK modulates cell behaviors essential for Kras oncogenesis (Xin 2024).
ERK signaling dynamics in hair follicle stem cells.
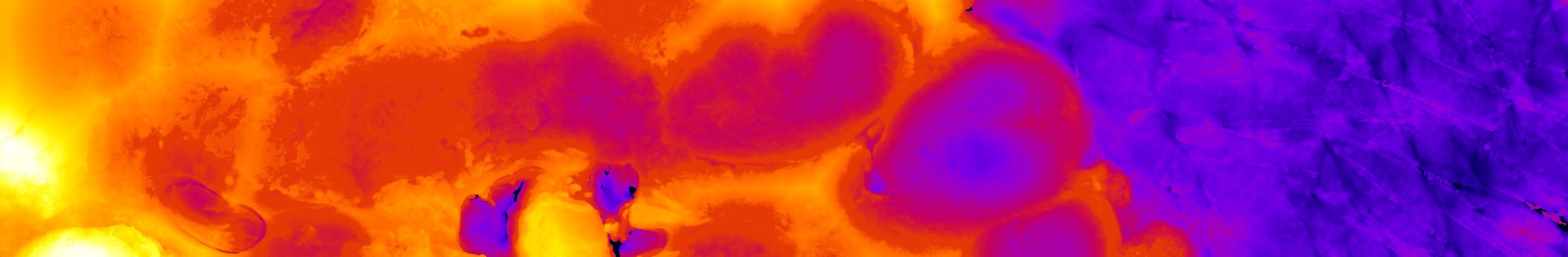
Redox imaging reveals oncogenic metabolic adaptations before competition outcomes become apparent
To understand the earliest molecular responses to the emergence of oncogenic cells in the skin, we have been adapting our live imaging approach to visualize fast-scale molecular states and events at a single-cell level before and after introducing oncogenic mutant cells. Recently, by tracking the metabolites NADH and FAD, we discovered that introducing either β-catenin mutant cells or Hras mutant cells leads to a rapid change in metabolic state of wild-type neighbors, but the “winner” cells in each model recover their metabolic state (Hemalatha 2025).
Two-photon images capturing the endogenous auto-fluorescence of the metabolic cofactors NAD(P)H and FAD at the granular and basal layer of the skin. Redox ratio (NAD(P)H/FAD) is represented as an intensity plot (scale on right).